Sodium borohydride/iodine reduction
Lately I’ve been doing quite a few asymmetric Michael additions using a chiral oxazolidinone, or one of the thio variants, as a chiral auxiliary. Some of the auxiliaries are commercially available but far too expensive for my taste. They can be easily synthesized from amino alcohols which are also commercially available but they too cost more then I’m willing to pay. So, the only option is to make them myself, which is not that bad a choice considering that the corresponding amino acids are not that expensive, especially the L ones. The D ones can be quite pricey. This is how the auxiliaries look like:
There are many ways to reduce amino acids to amino alcohols but my favourite by far is sodium borohydride/iodine combination (.pdf), which is not only non-expensive alternative but also convenient compared to, for example, lithium aluminum hydride (see, I don’t like burning labs). Below is how it looks like chemically written:
So, what is actually happening there? This is how I understand it:
First, iodine is reacting with one sodium borohydride, so you get borane, sodium iodide, and hydrogen iodide. Then hydrogen iodide is reacting with another sodium borohydride, so you get another borane and another sodium iodide, plus some hydrogen. All that borane is actually the stuff that reduces the acid to alcohol. Very nice and convenient. I’ve been doing it in 200 mmol scale and works just great, giving the amino alcohol in good >70% yield after recrystallization from toluene.
That’s it for today. Feel free to stop by again and comment if you like.
Explore posts in the same categories: Procedures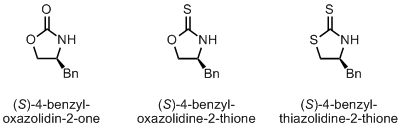


December 12, 2006 at 11:56 am
Not only is this a great route from a cost and safety perspective, but the workup is much easier, no nasty salts to monkey with, like in LiAlH4 reactions.
December 12, 2006 at 10:35 pm
I guess Ralph Salvatore has been using similar conditions, methyl iodide instead of iodine. He uses it on heterocycles. In a rather colourful garish slide show replete with mistakes, he gave a very good talk about the reduction of various heterocycles, i wonder if it would work under the current situation.
December 11, 2022 at 10:20 pm
mistakes such as?
December 15, 2006 at 1:27 am
I LOVE this reaction. The I2 oxidizes hydride giving a mole of H2 and generating BH3 in situ. I have reduced a lot of amino acids to the amino alcohol with this process. I fell into this reaction years ago after I had an LAH reduction of valine in THF go sideways on me, blowing the apparatus apart and splashing me with the reaction mixture. I tried warming the reaction with a warm water bath too soon and ended up paying for my impatience. I recall the taste and the sensation of H2 bubbling from my lips. Yikes. I did get in the habit of quenching LAH mixtures with powdered Na2SO4-10 H2O. Add xs hydrate and stir for 4 hrs or until the slurry is snow white. No fireworks and no silly Fieser workup to do. Filter, strip, and go have a beer.
December 17, 2006 at 1:46 am
Very nice protocol, and it brings up a point which I have wondered about for a while. Whenever I’ve used BH3/THF in the past, my product would always have residual THF signals in the NMR which were very difficult to purify away. Has anyone else come across this, and know what it is due to? I just moved on to a different reducing agent but this seems like a convenient method of producing BH3 in situ.
December 17, 2006 at 2:29 am
I think the active reagent is iodoborane actually, IBH2.
There is a nice alternative using a solution of 96%H2SO4 in THF dropwise added into a slurry od aminoacid and NaBH4 in THF at 0C. Worked very nicely for me with tert-leucine.
JR: are you sure it was THF signals and not butanediol? THF/Borane slowly goes bad because of a ring-opening reaction – although I don’t know how would this would produce butane1,4-diol in absence of a peroxide workup.
December 17, 2006 at 3:30 am
That would certainly explain the tenacity of the impurity…
December 17, 2006 at 7:36 pm
milkshake… can you elaborate a little more on that prep? is exactly as the I2 prep, but with 96% H2SO4? the reason i ask is because i will be doing a t-leucine reduction here soon and would like to try this! thanks.
December 18, 2006 at 5:56 pm
I don’t have the procedure with me anymore but if I remember it correctly (after nine years) it was as follows: tert-leucine+several molar eqivivalents of NaBH4 was suspended in THF, cooled on ice bath, an excess of 96% H2SO4 dissolved in THF (with cooling on water bath) was then very slowly added dropwise (using an addition funnel) to the borohydride-tert-leucine slurry over 1 hour with intense stirring on ice (gas evolution), the mixture was then stirred at RT overnight, pH adjusted with NaOH to about 10-11 and some saturated NaCl was added, the mixture was extracted several times with dichloromethane (the tert-leucinol is quite water-soluble). I think I took the crude tert-leucinol directly to the next step.
February 28, 2007 at 2:34 pm
i want to chloro to iodo reation cl is remove and iodine is attach which type of catlyst use with sodium iodide
March 29, 2007 at 1:38 pm
I had bad experiences with I2/NaBH4. 3,5-Dimethoxybenzoic acid was quantitatively destroyed, neither acid nor alcohol were recovered, the same thing happened with a substituted 4,6-Dihydroxy-2-pentyl-benzoic acid.
For reduction of beta-amino acids Zn(BH4)2 works great, >70%.
June 1, 2007 at 2:38 am
If borane is actual reductant, why not use borane directly? Is it more expensive?
July 14, 2007 at 6:37 am
Actually, sodium borohydride and sulfuric acid works as well, if you’re not a fan of iodine.
September 21, 2007 at 3:34 pm
I found that by first making the ethyl ester from the amino acid and refluxing it in 50% ethanol/water for 4.5.hours using 4 equiv of Nabh4 with yields 81% to be best since it doesn’t require any I2 or THF
December 20, 2007 at 6:46 pm
Hi @all,
nice write-ups, could anyone tell about the work-upo procedure a little bit of the Bh3*THF?
I would filter the salts of NaI and then simply strip off the solvent and do an acid base.
Does one need to boil the mixture after strippig the solvent?
April 22, 2008 at 5:12 am
I did the same like java-reduction of ethyl esters to alcohols using NaBH4 alone (in ethanol)…
April 1, 2009 at 9:30 am
The THF traces in the reaction mixture can be easily removed by taking product in organic solvent and giving it a water wash. The organics is then dried over anhydrous sodium sulphate and evaporated, dried further over vaccum for about half an hour under warm heating. This is rally a good way of doing this reaction…one naother option is use BH3/DCM as an alternate of BH3/THF(dry).
Thanks & Regards,
May 17, 2009 at 5:35 pm
Dear sir,
I tried reduction 0f Phenyl alanine with NaBH4(1.0 eq) in THF and H2So4(1.25eq) in ether,but i didnt get the product,and also i t is difficult to check tlc after fomation of BH3 comlex.
November 15, 2015 at 3:53 pm
I tried same reaction in sulphuric acid medium 70% yield.
December 8, 2016 at 5:59 pm
Dear could you plz provide experimental details with workup.
Thanks.
July 1, 2009 at 4:29 am
But how does one reduce an amino alcohol, to an amine?
Any refs would be great!
September 16, 2009 at 2:00 am
You would react the alcohol with thionyl chloride (SOCl2), or PBr3 to create the alkyl halide (R-X). The X (Halogen; Cl or Br)is then replaced using any number of hydrogenation methods (LAH, H2& Ni, H2 & Pb).
My question is simple; Has anyone any information on any CNS effects of the compound given above as an example (the reduction product of phenylalanine – referred to as phenylalinol, or alpha-methoxy phenylethylamine?)
July 29, 2011 at 6:46 pm
“you would react the alcohol with thionyl chloride (SOCl2), or PBr3 to create the alkyl halide (R-X). The X (Halogen; Cl or Br)is then replaced using any number of hydrogenation methods (LAH, H2& Ni, H2 & Pb).
My question is simple; Has anyone any information on any CNS effects of the compound given above as an example (the reduction product of phenylalanine – referred to as phenylalinol, or alpha-methoxy phenylethylamine?)”
….How about using NaBH4 /NiCl since the catalyst would be the NiCl and NaBH4 would generate the Hydrogen, also,
“Transitional metal compound mediated reductio of alpha -amino acids to 1,2-amino alcohols with nabh4 in water”
….Turk. J. Chem, 1999, 23, 123-126
The above study is much better without having to first synthesise the ester as in the study…
“Studies of opticall active amino acids synthesis of optically active alpha-aminoalcohols by the reduction of alpha-amino acids esters by sodium borohydride”
…Chem. Pharm. Bull. 1965, 13,(8), 995-1000
…..and so I wonder if NaBH4 /NiCl would dehalogenate the now halogenated former primary aliphatic alcohol …….any comments with some references please….java
December 8, 2009 at 4:48 am
I am trying to reduce an pyrimidin ester / pyrimidin acid but no common reducing agent works well, always getting over reducing product, is there any one who did reduction of ester of acid having pyrimidin moiety??
January 18, 2010 at 10:06 pm
Hello, I’m trying to reduce an nitrile ( phenoxyacetonitrile moiety) in the presence of pyrimidine group.Moreover i have problems with the solubility of the compound…in diethyl ether it is not soluble, in THF: 50mg in 5ml…I tried the reduction LAH/THF but it didn’t give me good result. :(.Do you have any idea for a reducing nitrile agent in the presence of pyrimidine moiety? The nitrile group is very reactive and in the in case of pyridine derivatives the nitrile group reacts with LiAlH4 a 0°C in only 10 minutes? Is the problem the pyrimidine or the solubility of the compound…Thank you very much.
January 22, 2010 at 12:05 pm
this reaction is very helpful
February 5, 2010 at 9:28 am
u can try LiBH4
July 3, 2010 at 8:44 am
how can we reduce nitrile group follwing same approach
July 3, 2010 at 8:45 am
plz do imform if method available
January 9, 2011 at 7:17 am
Can anyone suggest how to reduce cyclic imines?
March 18, 2011 at 9:30 am
please tell the mechanism when reduction of imine by sodium borohydride in ethanol with few drops of sodium hydroxide. what is the purpose of sodium hydroxide.
March 18, 2011 at 9:31 am
please tell the mechanism when reduction of imine by sodium borohydride in ethanol with few drops of sodium hydroxide. what is the purpose of sodium hydroxide
July 29, 2011 at 6:47 pm
….remember that nabh4 works best under an non acidic environment…java
October 28, 2011 at 9:11 pm
Has anyone tried reduction of tryptophan? I’ve done several methods including borane in situ from iodine/sodium borohydride and sulphuric acid in ether/sodium borohydride in THF. Both produced little yields.
November 7, 2011 at 3:56 pm
Tryptophan is the only amino acid having indole side chain so this ring always cause steric hindrance to the hydride nucleophile, better to try BH3-DMS (May be work well)
November 26, 2011 at 6:14 am
hellow sir cyno to amine can i do in sodium borohydred/ sulphric acid?
January 30, 2012 at 6:32 pm
I personally disagree with the idea that I2 + NaBH4 gives BH3. The original paper states that refluxing a boc amine in NaBH4/I2 does not reduce the boc, which would definately get reduced by BH3 under reflux in THF
June 3, 2023 at 11:26 pm
The paper: Selective Reduction of Carboxylic Acids into Alcohols Using NaBH, and I2
TableI. SelectiveReductionsofCarboxylicAcidsto Alcohols”
J. V. Bhaskar Kanth and Mariappan Periasamy* School of Chemistry, University of HydeRabad
Uses a different explanation of the mechanism.
NaBH, + RCOOH -> RCOOBH3Na + H2
RCOOBH3Na + I2 -> RCOOBH2+ 0.5NaI + 0.5H2-> RCH2OBO
The selectivities realized with the NaBH4/12system over the borane reagents such as BH3-THF deserve an expla- nation. Hydroboration of olefins with the RCOOH/ NaBH, system is relatively slow compared to hydro- borations using BH3-THF.8*s Also,the rates of reaction of cyclohexene and caproic acid with diborane are com- parable.'” ….
Check the reference for more info. It seems to suggest transient diborane is the reduction agent.
April 6, 2012 at 2:33 am
In non-coordinating solvents like heptane, it’s pretty clear that I2 and MBH4 (M = Li, Na, K) react to give borane and mixed iodoboranes. The reaction has been investigated as a way to make boron triiodide. To complete the reduction of iodine, ~10 equiv. KBH4 need to be used. Some of this doesn’t react, but when the effluent gasses of the reaction are passed through water, boric acid is among the hydrolysis products.
Briggs, A. G.; Simmons, R. E. Naturwissenschaften 1990, 77, 595–597.
April 6, 2012 at 10:57 am
dance competition, dance competitions,showstoppers dance competition,headliners dance compition,starquest dance competition,starbound dance competition,list of dance competitions,dance competitions 2012,revolution dance competition,hall of fame dance competition, vip dance competition,kar dance competition Thanks for that awesome posting. It saved MUCH time 🙂
April 12, 2012 at 8:12 am
it is very good concept.
May 29, 2012 at 7:57 am
I try to find this method, so can you give me the experimental flow chart for reducing amino acid, such as glycine, methionine etc.
I had read this from many journals but I still not cleared in the experimental method.
Thanks in advance.
October 4, 2012 at 9:09 am
Phenylalanine is necessary for the neurotransmitter production in our brain. It also helps with anxiety and depression by synthesizing serotonin which calms the mind and makes us feel good. .”;*.
With appreciation
http://www.healthmedicinelab.com/symptoms-of-mono/
May 18, 2013 at 4:38 pm
I know this website presents quality based content and other material, is there any other
web site which presents these things in quality?
May 30, 2013 at 11:35 am
The process of car shopping can be lengthy. You have so many options, not to mention things to consider as you compare each one.
You will be able to make the right decision if you know enough about
car shopping. Here are some great tips on buying a car.
May 31, 2013 at 4:45 pm
As age progresses, our memories commence to dwindle. It has been said that the memories lost are
replaced with new memories, but that is not comforting.
Use the methods you just study about numerous things.
June 17, 2013 at 7:49 pm
Hi colleagues, its great piece of writing concerning cultureand fully explained, keep it up all the time.
June 27, 2013 at 4:12 am
Thanks for another fantastic article. Where else may
anybody get that kind of info in such a perfect approach of
writing? I’ve a presentation next week, and I am on the look for such information.
September 25, 2013 at 11:34 am
Hi colleagues, its enormous paragraph on the topic of
tutoringand entirely defined, keep it up all the time.
October 26, 2013 at 12:12 pm
I am sure this article has touched all the internet viewers,
its really really nice post on building up new website.
September 23, 2014 at 9:51 pm
Today, I went to the beach with my children. I found a sea shell and gave it
to my 4 year old daughter and said “You can hear the ocean if you put this to your ear.” She placed the shell to her ear and screamed.
There was a hermit crab inside and it pinched her
ear. She never wants to go back! LoL I know this is completely off topic but
I had to tell someone!
June 9, 2015 at 9:35 am
Interested in trying the sulphuric acid and sodium borohydride version. What safety issues need to be accounted for?
November 15, 2015 at 3:58 pm
I did in sulphuric acid medium and i got 70% yield.workup also easy and isolayion also easy.
December 8, 2016 at 5:46 pm
I would really like if NaBH4-I2 method can be replaced with LAH. Have you tried reducing Tryptophan? As It is not soluble in THF in reasonable volume.
I could not open pdf file in the text. Could you plz respond.
Thanks.
November 10, 2017 at 8:45 pm
Hi!
great method, i know. my colleagues used this for the reduction of valine, l phenyl glycine but i don’t know what the heck for my case- in reducing l phenylalanine. when i have added MeOH ( FOR QUENCHING REACTION- REACTANTS: IODINE,NaBHO4 in thf) after 16 hr reflux, reaction mixture got iodinish! then i added KOH BUT my compound got stuck in aqueous layer nothing extracted in chloroform/ DCM or even in IPA and chloroform mixture, also tried to acidify but was of no use. my supervisor said you must had done something wrong.
how to fix, or what has done with it?
February 20, 2018 at 12:11 am
I do not even understand how I finished up
right here, however I believed this post used to be good. I don’t
recognize who you’re but certainly you’re going to a well-known blogger when you aren’t already.
Cheers!
October 15, 2020 at 3:17 pm
Great post! We are linking to this particularly great post on our website.
Keep up the great writing.
December 13, 2022 at 11:38 am
pls tell process for reduction of carboxylic acid with sodium borohydride /HCl of NaBH4/H2SO4