Paul at ChemBark was writing that “the Aldrich catalog ranks #1 on my list of the top desk references of all time”. I agree, it’s a great help when looking for melting/boiling points, densities, etc. However, I have a computer 2 meters from my hood and 1 meter from my desk, so the Sigma-Aldrich website is what I mostly use.
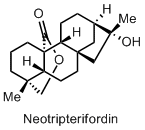
I also have another nice desk reference. It’s the total synthesis paper of Neotripterifordin by Corey and Liu (JACS, 1997, 119, 9929-9930). Actually my desk reference is the supporting information of that paper, which is very well written and contains many common reactions.
The reactions on that paper are, for example:
LiAlH4 reduction
R-OH -> R-I
Wittig salt preparation/Wittig reaction
acetylation/deacetylation
silyl protection/deprotection
mCPBA oxidation
THP protection/deprotection
MnO2 oxidation
Katsuki-Sharpless epoxidation
benzylation
Dess-Martin oxidation
Wolff-Kishner reduction
OsO4 oxidation
Pd/C catalyzed hydrogenation
Birch reduction
[2+2] cycloaddition
ozone oxidation
DIBAL-H reduction
Barton-McCombie reaction
Oh, and this is how Neotripterifordin looks like:
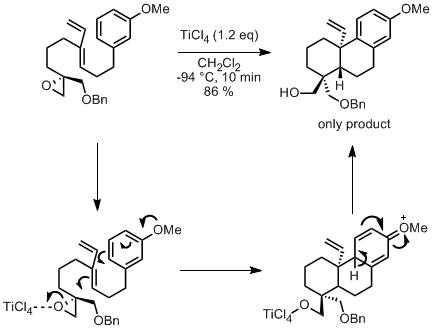
There is a nice cascade reaction in that synthesis:
Interesting paper to read. I recommend.


Recent Comments